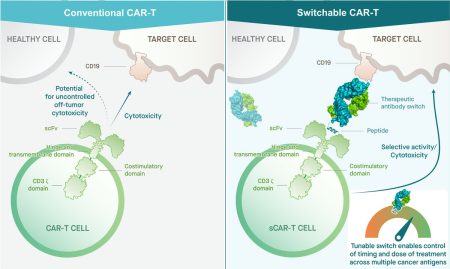
Switchable CAR-T (sCAR-T) Platform
The Calibr-Skaggs Institute for Innovative Medicines is developing a controllable, universal cellular therapy platform (sCAR-T) as a next-generation CAR T-cell therapy to address the challenges of treating more diverse, solid tumor cancers. sCAR-T has applications in autoimmune diseases such as lupus as well. The precise control afforded by our switch technology is designed for higher efficacy, safety, durability and universality.
Unmet Medical Need
Cellular immunotherapy harnesses a patient’s immune system by taking T cells from a patient, genetically altering them outside the body, and returning them to the patient where they target cancer cells directly. Traditional CAR-T has been highly successful, abolishing tumors in up to 90% of certain blood cancer patients who failed all other treatment options. However, various barriers including off-target toxicity, T-cell ‘exhaustion’ (weakening of CAR-T cell response over time), and manufacturing challenges restrict the widespread use and application of traditional CAR-T to solid tumors.
A MORE ROBUST, VERSATILE AND SAFE CANCER TREATMENT
Our switchable CAR-T platform goes beyond approved CAR-T cell therapies by delivering controllable, safe and persistent treatment regimes. Our platform features a universal design leveraging antibody-based switches that can be targeted to multiple antigens on tumor cells–increasing specificity while reducing potential off-target effects. sCAR-T has already demonstrated the ability to completely eradicate multiple types of tumors in preclinical mouse models with durable and controlled responses (Rodgers, et al 2016 PNAS). This highly differentiated approach affords the opportunity to treat potentially limitless varieties of cancers in the clinic—including solid tumors—with improved safety.
Clinical Progress
We have completed a phase 1 clinical trial (NCT04450069) to evaluate the safety of the platform (CLBR001) and a CD19-targeted switch (SWI019) for B-cell malignancies. This therapy received Fast Track designation from the FDA in October 2020, which enables enhanced access to the agency to potentially accelerate the development and review of novel therapies. Initial results announced underscore the potential of this therapy.
We have also received FDA clearance to test the CLBR001+SWI019 therapy in B-cell-driven autoimmune diseases, with the phase 1 clinical trial expected to begin soon (NCT06913608). Using the same CLBR001 platform, we developed a novel switch to target metastatic breast cancer. We are currently enrolling patients to test the safety of this breast cancer therapy in phase 1 clinical trials (NCT06878248). Interested patients can learn more on the National Institutes of Health (NIH) clinical trials portal.
Key Publications
Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang, Singer O, Ma J, Nunez V, Shen J, Woods AK, Wright TM, Schultz PG*, Kim CH*, Young TS*. (2016) Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proceedings of the National Academy of Sciences of the United States of America. Jan 26;113(4): E459-68.
Viaud S, Ma JSY, Hardy IR, Hampton EN, Benish B, Sherwood L, Nunez V, Ackerman CJ, Khialeeva E, Weglarz M, Lee SC, Woods AK, Young TS*. (2018) Switchable control over in vivo CAR T expansion, B cell depletion, and induction of memory. Proceedings of the National Academy of Sciences of the United States of America. Oct 29; 115 (46):E10898-E10906
Press
Calibr Announces Promising Results from Phase 1 Study of switchable CAR-T for B Cell Malignancies (Sept 21, 2022) Read More
Calibr Announces Preliminary Clinical Data (Dec 8, 2021) Read More
Calibr receives The Conrad Prebys Foundation Grant (Mar 23, 2021) Read More
FDA grants Fast Track designation (Oct 1, 2020) Read More
FDA clearance of Investigational New Drug application (Feb 3, 2020) Read More
AbbVie and Calibr announce collaboration for next-generation T-cell therapies (Jun 25, 2018) Read More
Have questions or feedback for our team?



